Arsenopyrite dissolution is an important source of arsenic’s environmental occurrence and is well-studied under oxidative conditions. Although O2 is considered the main oxidant for the oxidation of arsenopyrite in earth’s surface environments. Previous studies have found that arsenopyrite has a higher extent of oxidation in the presence of water and oxygen simultaneously. An effective strategy to unveil the role of water in arsenopyrite dissolution is to examine the mineral-water interaction at anoxic conditions. However, little research is available concerning this process despite the potential of arsenopyrite dissolution to be a source of arsenic contamination in oxygen-deficient environments such as confined aquifers. The goal of this study is to determine the pH dependence of arsenopyrite dissolution under anoxic conditions in terms of speciation, reaction rate, and stochiometry, and ultimately shed light on the mechanism of arsenopyrite-water interaction. The objective will be achieved through batch and flow-through experiments at various pH conditions, in conjunction with aqueous and surface speciation measurements. The principal findings of this study can be succinctly outlined as follows:
(1) The flow-through experiments showed that anoxic dissolution rates of arsenopyrite exhibited a positive dependence on pH, contrary to the reported behavior of arsenic release in oxygenated water (Figure 1).
(2) Long-term dissolution data revealed mostly stoichiometric release of As and S; however, the cation to anion ratio in solutions decreased with decreasing acidity in the pH range of ~2.0 to ~5.7, presumably resultant from surface retention of Fe3+ via ferric hydroxide formation at circum-neutral to alkaline conditions, consistent with XPS observation of oxidized iron Fe(III)-O on the dissolved surface (Figure 2).
(3) XPS also detected the presence of S(-II) and oxidized S and As surface species such as arsenate and sulfate, suggesting the anoxic dissolution is accompanied by electron transfer processes. The detection of H2O2 in our speciation analyses confirmed this hypothesis and led us to propose a dissolution mechanism on the basis of surface species auto-redox reaction and surface complexation model (Figure 3).
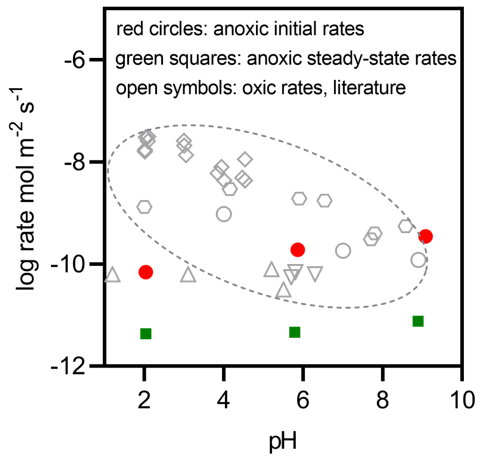
Figure 1 Anoxic dissolution rates of arsenopyrite at different pHs measured in the present study juxtaposed with published oxidative dissolution rates.
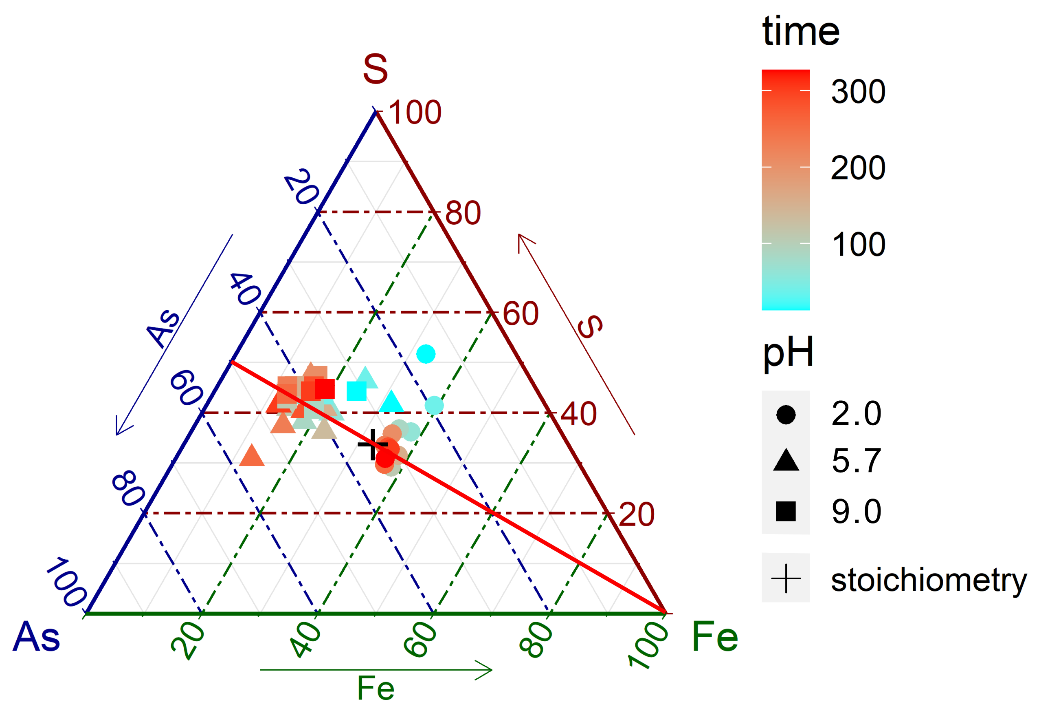
Figure 2 A ternary-component phase diagram showing the effect of pH on the relative amount of Fe, As, and S released in long-term dissolution
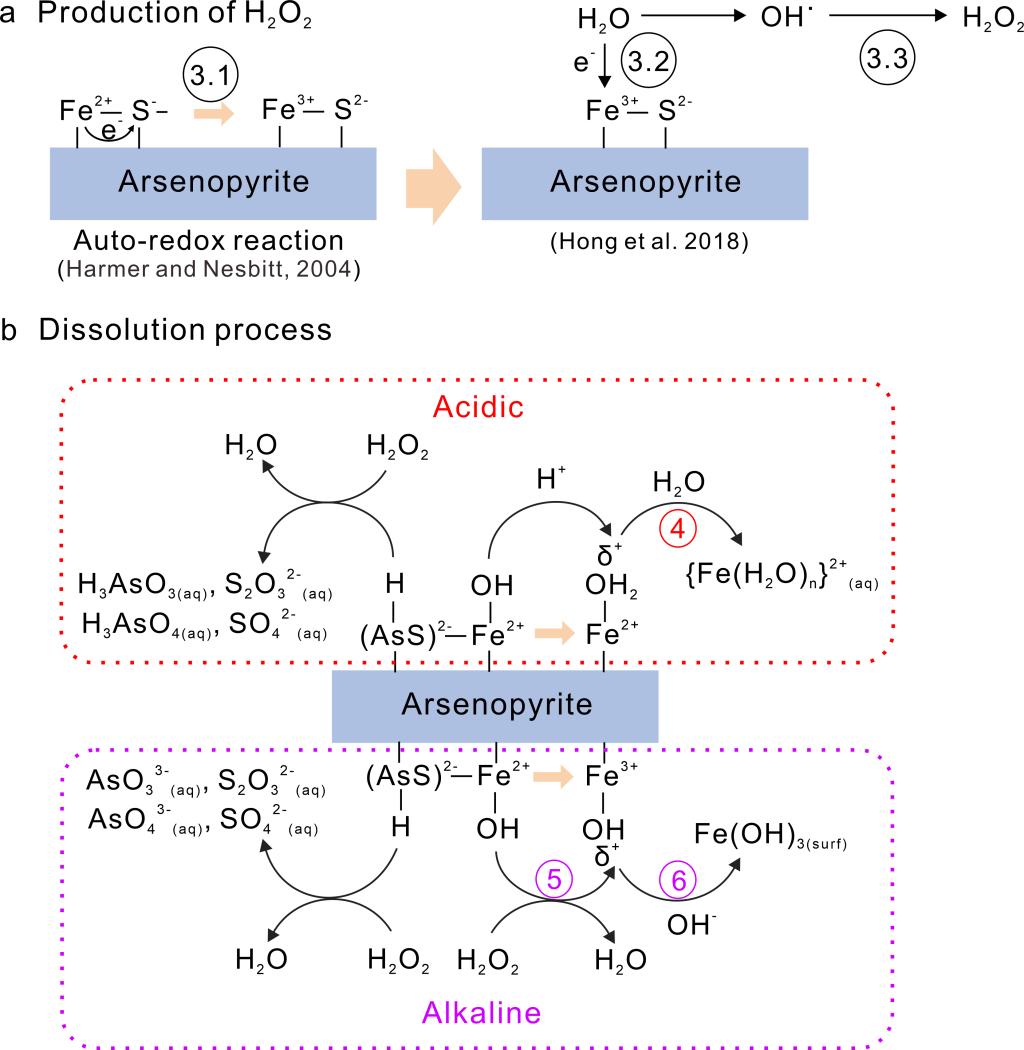
Figure 3 A schematic diagram showing the possible mechanism of anoxic dissolution of arsenopyrite.
Link of article: https://doi.org/10.1016/j.gca.2023.10.030