Arsenic is a highly contaminated element, causing environmental and health effects in numerous worldwide locations. The dissolution and precipitation of arsenic minerals control the activity of arsenic in the environment. Hence, study of the related thermodynamics and kinetics of the dissolution and precipitation of common arsenic minerals is of great significance for quantitatively understanding the migration and transformation of arsenic in the environment. Calcium arsenate minerals are ubiquitous in the environment and important arsenic-fixing minerals. However, there is little reliable thermodynamic and kinetic parameters for assessing its stability. The present study intends to investigate pharmacolite formation initiated by heterogenous nucleation through in situ observations of the mineral-water interface. Experiments were performed by exposing gypsum substrate to solutions supersaturated with respect to pharmacolite followed by atomic force microscopy imaging to record changes in surface morphology and topography. The data are used to determine growth mode, step speed, and the rate of step birth. The main conclusions of this study are summarized as follows:
(1) Pharmacolite crystallization is found to proceed via layer-by-layer growth mode initiated by 2-D surface nucleation or at spiral dislocations (Fig. 1, Fig. 2) in accord with the classical crystallization model;
(2) Subsequent theoretical analyses allow to determine step energies and kinetic coefficients in major growth directions on (010). The results depict a highly anisotropic nature of pharmacolite with direction-specific step energies and kinetic coefficients for at least four crystal orientations. In specific, [001] steps are shown to be the most stable and the slowest advancing, and hence the dominant morphological expression, consistent with observations at both static and flow-through conditions. In contrast, the development of [100] and [101] directions appears to be saturation dependent because the latter is energetically favored (i.e smaller step energy) while the former kinetically promoted (slower growth rate at lower supersaturation);
(3) Gypsum plays an important role in aiding pharmacolite formation through epitaxy as the two minerals share a range of structural commonalities. The notably reduced supersaturation needed in substrate-assisted pharmacolite crystallization relative to bulk solution nucleation suggests gypsum may significantly reduce the energy barrier for the mineralization reactions and hence may find applications in As remediation practice.
Zhu, Xiangyu., Chang, Pei., Zhang, Jianchao., Wang, Yuebo., Li, Siliang., Lu, X., Wang, R., Liu, Cong-Qiang. and Teng, H.H. (2022) Kinetics and energetics of pharmacolite mineralization via the classic crystallization pathway. Geochim Cosmochim Ac 339, 70-79. https://doi.org/10.1016/j.gca.2022.10.039
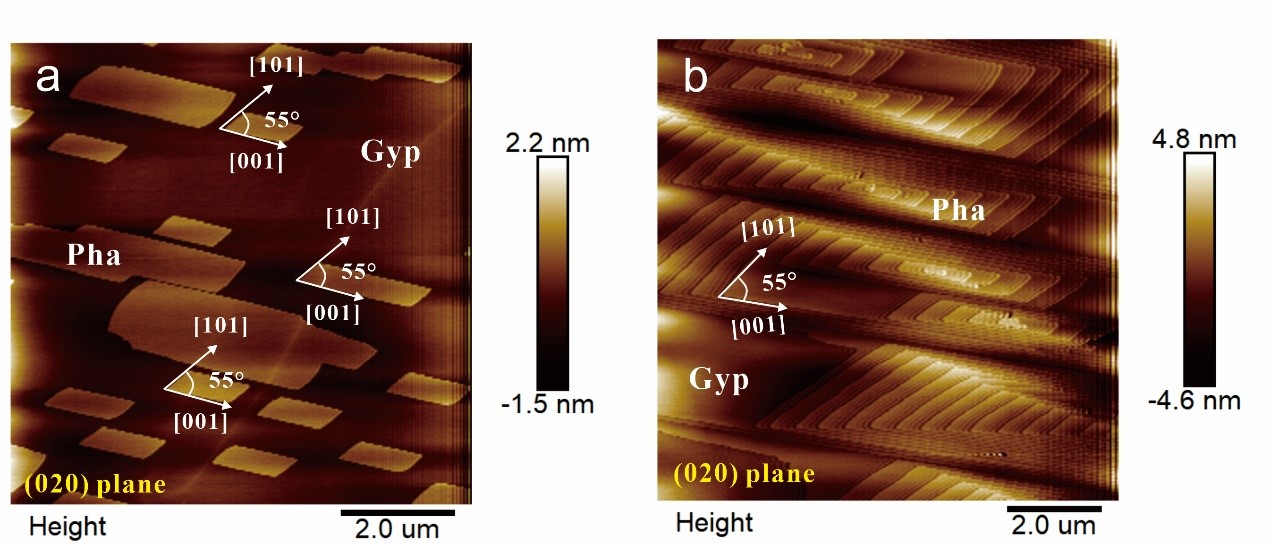
Fig.1. (a) AFM Height image of pharmacolite 2-D monolayer growth on gypsum (010) surface. (b) AFM Height image of pharmacolite 3-D hillocks developed on gypsum (010) surface.
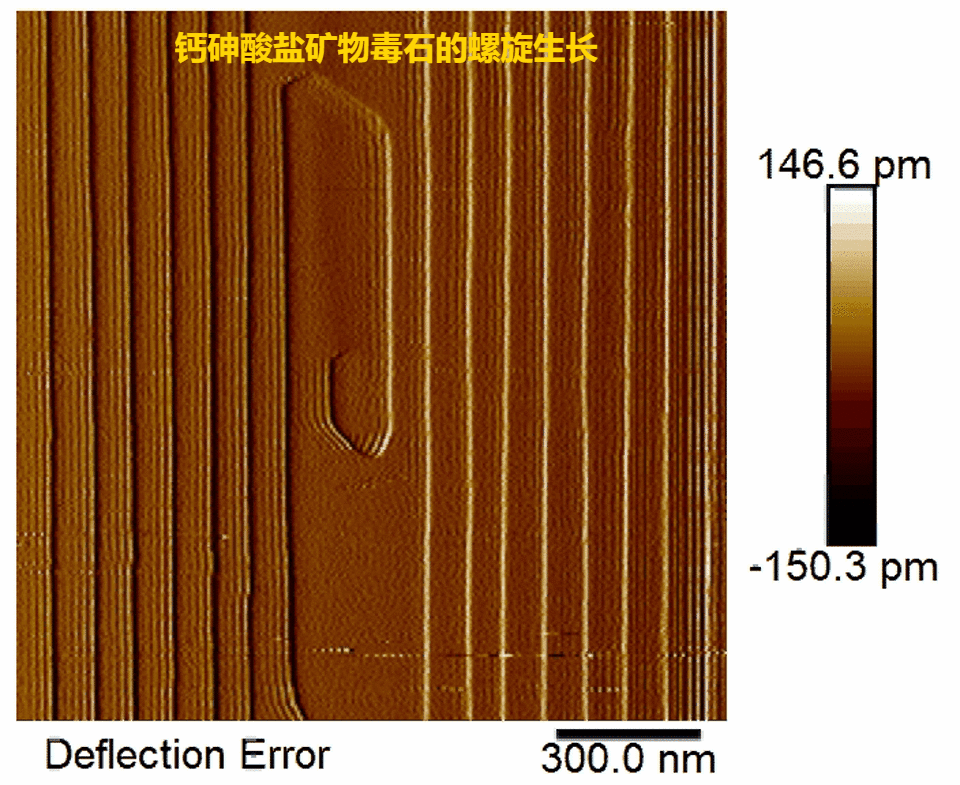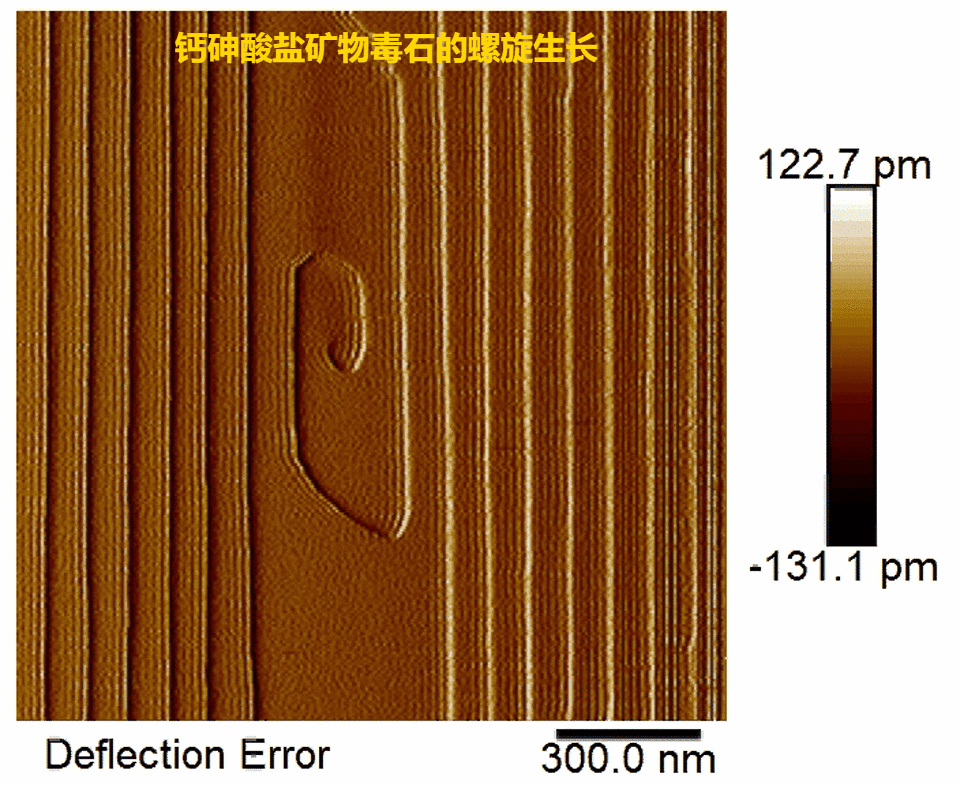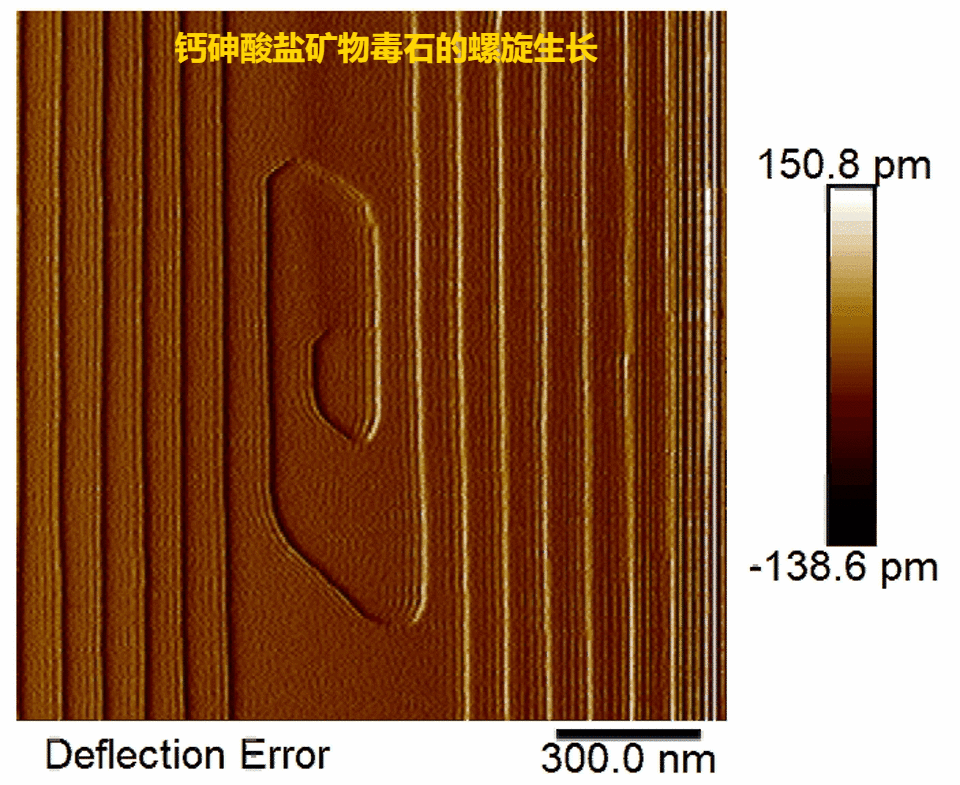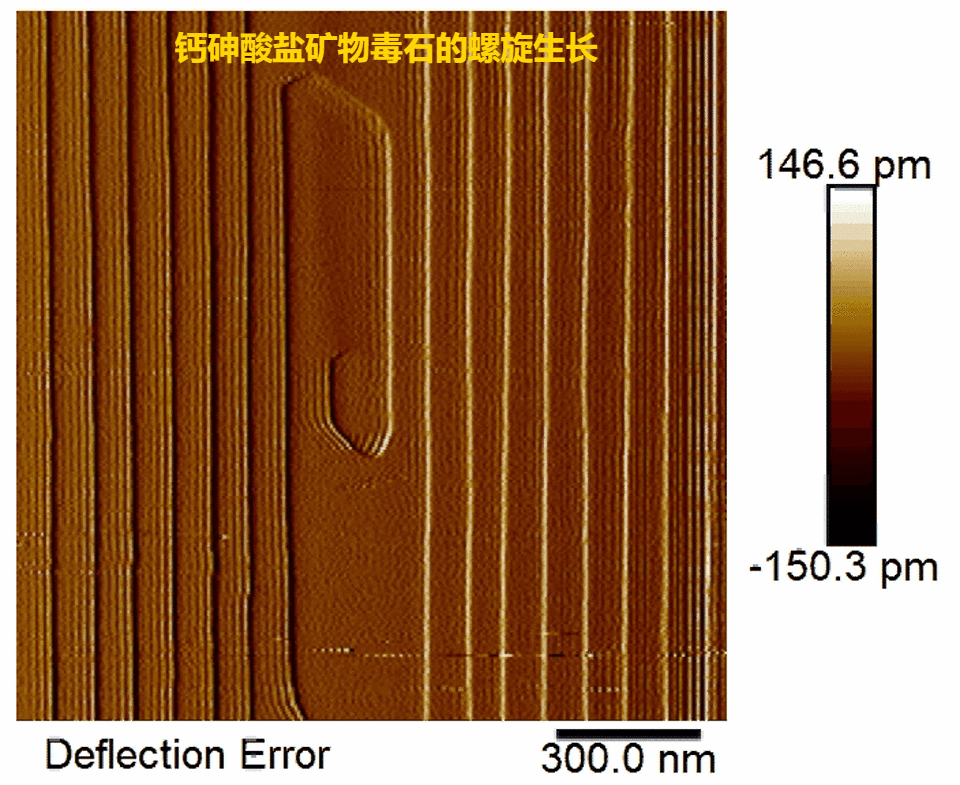
Fig.2.Real-time observations of pharmacolite spiral growth