Mercury (Hg) is a global pollutant with significant toxicity for life. Ocean, one of the largest reservoirs of Hg on Earth, covering 71% of earth surface with a huge volume (V=1.34´1021 L), poses a significant impact on the global Hg cycle. However, traditional techniques based on Hg concentration analysis cannot track different biogeochemical processes in marine systems accurately because Hg concentration is unable to discern different biogeochemical processes and to track its sources. Hg isotopes demonstrated the potential application to track the sources and the processes in various ecosystems. Though, seawater Hg isotope data are rarely reported.
It is difficult to measure Hg isotopes in seawater because current methods cannot quantitatively extract dissolved Hg from seawater due to the extremely low Hg concentration (around 0.2 ng/L) and the high interference of matrix (e.g. OM, high salinity, I-) in seawater. Here we introduce a new coprecipitation method to preconcentrate Hg from dilute seawater for accurate Hg isotope measurement. Theoretical calculation shows that the coprecipitation as Hg sulfide is appropriate for purifying Hg from seawater matrix. The coprecipitation was achieved by sequential addition of 0.5 mL of 0.5 M CuSO4, 1 mL of 0.5 M Na2S and 1mL of 0.5 M CuSO4 reagents, which allowed for quantitatively precipitating Hg from up to 10 L seawater. Then, the method is validated by the quantitative Hg recovery and the good reproducibility of long-term measurements of Hg isotope ratios in synthetic solutions spiked with well-calibrated standard materials. Repeated measurements of synthetic seawaters spiked with certificated standard materials using the entire method gave near-quantitative Hg recovery of 98±12% (n=32, 2 SD) and relatively low procedure blank (103 pg of Hg, n=8). And the results give identical Hg isotope ratios with the average δ202Hg values of -0.02±0.14‰ (n=21, 2 SD) for NIST 3133 and -0.58±0.12‰ (n=11, 2 SD) for NIST 3177, respectively, consistent with the calibrated values, suggesting no isotope fractionation during the preconcentration.
A total of 6 nearshore seawater samples from the Yellow Sea and Bohai Sea (China) were analyzed using the coprecipitation method. The data showed large fractionation of Hg isotopes, and revealed possible impact of both atmospheric and anthropogenic inputs to coastal seawater Hg budget, implying the potential application of this method in studying marine Hg systematics and global Hg cycling.
The paper was published online in the journal Analytical Chemistry (IF=6.798) under the title “Coprecipitation of mercury from natural iodine-contained seawater for accurate isotope measurement” on November 22, 2021. Yulong Liu, a doctor is the first author. The co-corresponding author is Professor Jiubin Chen from Tianjin University.
Paper link: https://pubs.acs.org/doi/10.1021/acs.analchem.1c03060.
Professor Jiubin Chen’s team (the Center for Advanced Science of Isotopes (CASI) at Tianjin University) is devoting to the advanced research domains of the stable isotope geochemistry, with a focus on the geochemistry of metal stable isotopes, with on one hand, developing measurement methods for new metal stable isotope systems (such as Ga, Sn, Sb), and on the other hand, improving the existing theoretical framework of metal stable isotopes (such as Hg, Cu, Zn, Fe, Li, Mg, Ba) and expanding its application in surface earth system science. In addition to provide the fundamental technical support for various fields of surface earth system science, the CASI aims to make breakthroughs and innovations in the basic theory, measurement technologies and application areas of isotopes.
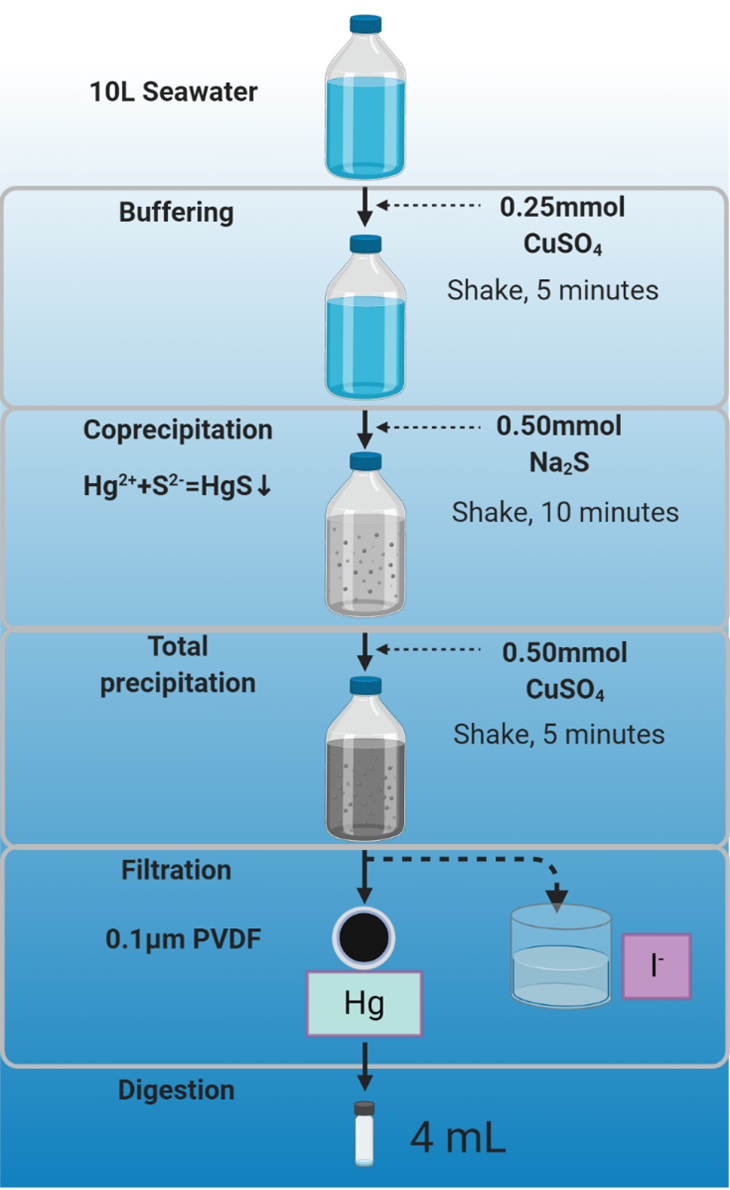
Figure 1. Scheme of coprecipitation method for 10 L seawater samples